ARCA Technology
We make life worth living through
innovation and commitment
innovation and commitment
Technology
ARCA Technology
ARCA Technology
ARCA Technology: Obtaining optical pure amino acids using ARCA
that binds amino acids through imine formation
that binds amino acids through imine formation
ARCA
imine
Amino Acid
ARCA forms an imine with amino acids at basic condition. Hydrogen bonding between N of imine and OH, along with hydrogen bonding between the urea proton and the carboxylic anion take place. Depending on the optical properties of α-amino acids, the steric hindrance between Alkyl position of α-proton and H of imine may occur which affects the stability.
S-ARCA, as seen in the picture below, exhibits a higher stability with D-amino acids due to less steric hindrance. The NMR pattern shows that the stronger hydrogen bond between the urea proton group and the carboxyl group may contribute to greater receptor stereoselectivity for binding D-amino acids.
Applying this mechanism, ARCA can be an effective extractant for the resolution of racemic amino acids, and can convert amino acids from an L- to D-form or from a D- to L-form at organic basic condition.
S-ARCA, as seen in the picture below, exhibits a higher stability with D-amino acids due to less steric hindrance. The NMR pattern shows that the stronger hydrogen bond between the urea proton group and the carboxyl group may contribute to greater receptor stereoselectivity for binding D-amino acids.
Applying this mechanism, ARCA can be an effective extractant for the resolution of racemic amino acids, and can convert amino acids from an L- to D-form or from a D- to L-form at organic basic condition.
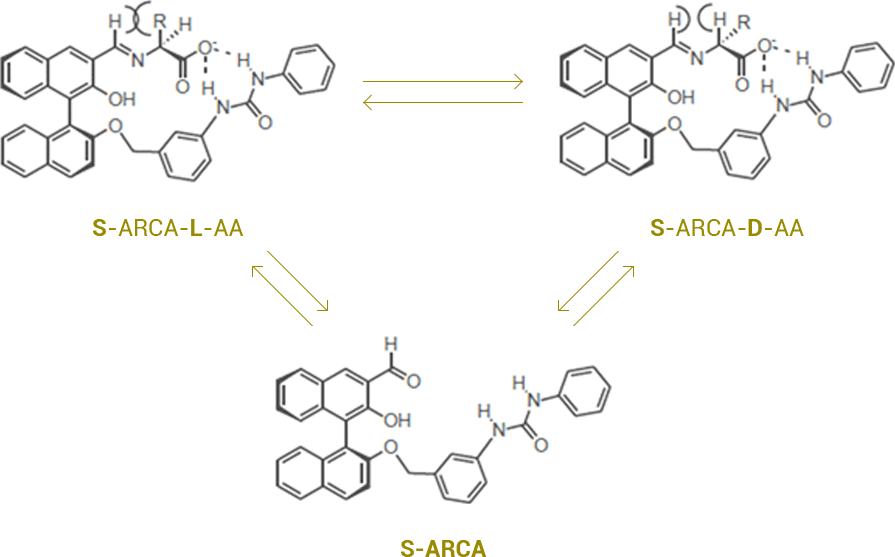
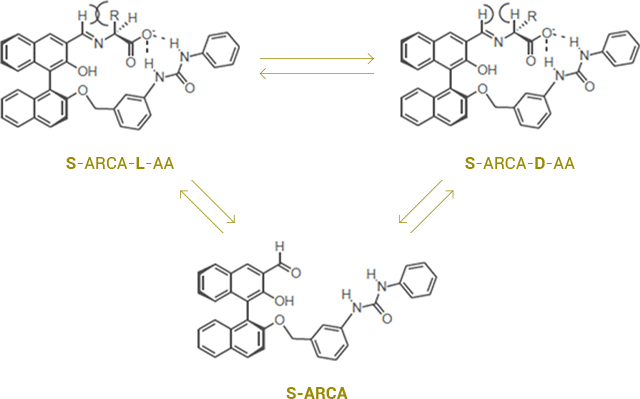
Table 1. The Ratio of (1-D-aa) to (1-L-aa) at Equilibrium
| amino acids | D/L ratio | amino acids | D/L ratio |
|---|---|---|---|
| threonline | 20/1 | methionine | 11/1 |
| glutamine | 15/1 | glutamic acid | 11/1 |
| histidine | 14/1 | serine | 11/1 |
| arginine | 14/1 | leucine | 9/1 |
| asparagine | 13/1 | tryptophan | 8/1 |
| tyrosine | 12/1 | alaine | 7/1 |
| phenylalanine | 11/1 |

